NTK Panel
NTK assays are currently offered for cerebrospinal fluid (CSF) and/or blood
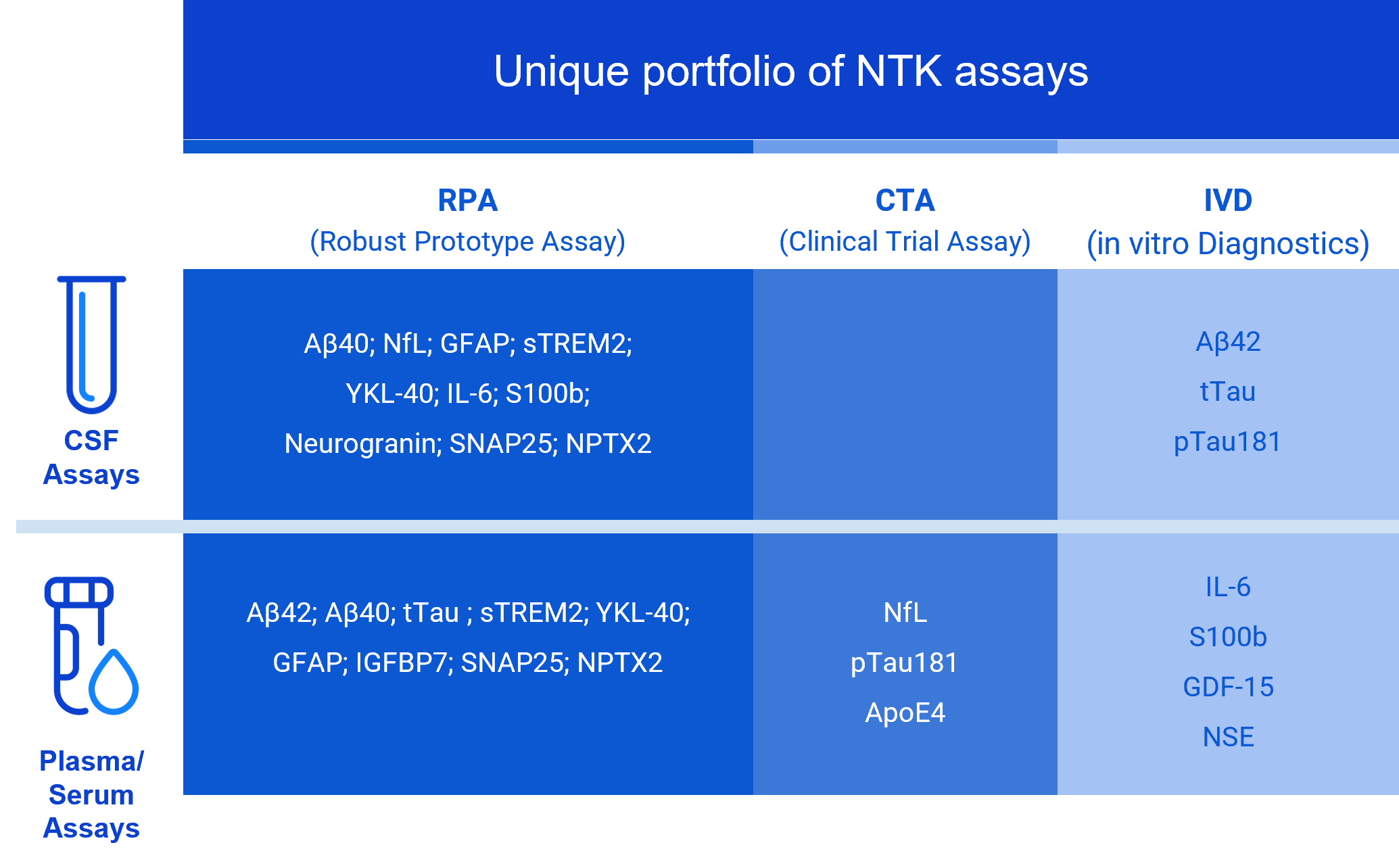
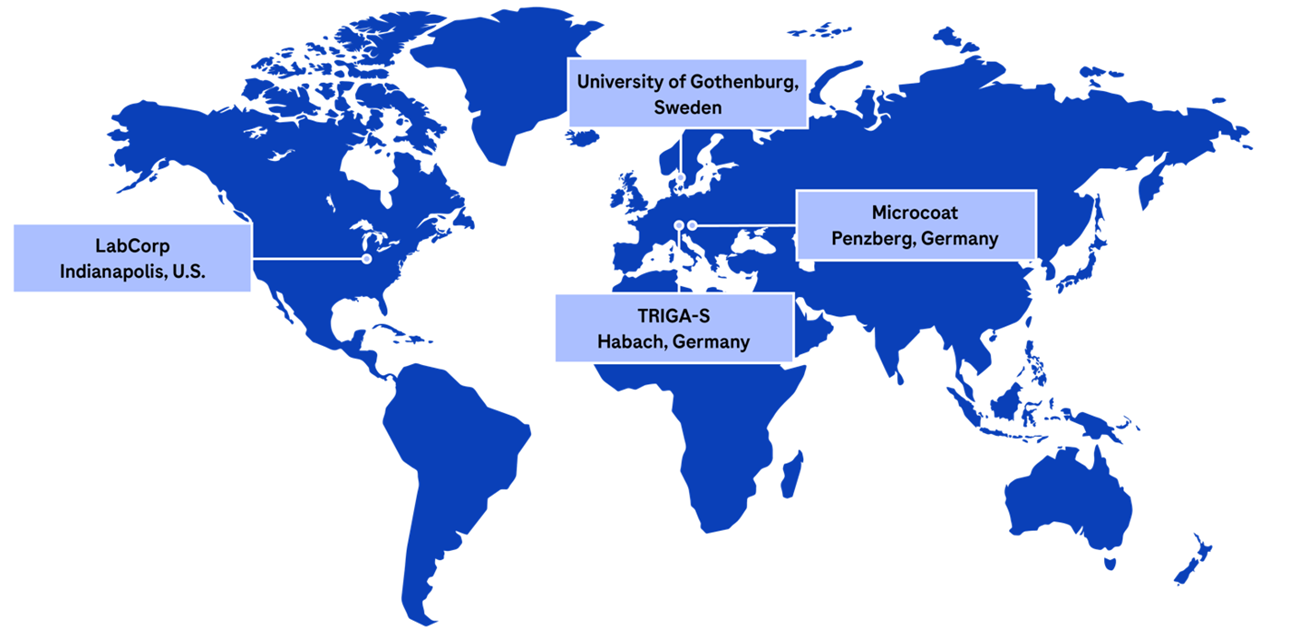
The NTK portfolio is measured in four measurement sites
The power of Elecsys® in Alzheimer's disease
Robust and accurate biomarker tests to solve clinical questions
- Elecsys® assays achieve highly accurate and precise results across all Cobas® platforms, with the precision being confirmed in the Alzheimer's Association Quality Control (AAQC) program;
- Elecsys® assays show robust reagent and calibration stability.
To learn more about the Power of Elecsys, please click here.
