The NeuroToolKit
The NeuroToolKit (NTK) is a collaborative initiative across academia, industry and philanthropy partners to generate high quality, reproducible and comparable biomarker data. Together, the NTK partners aim to advance research and inform developments in diagnostic and treatment solutions in AD, neurodegeneration and other neurological disorders.
The NTK aims to advance research through
(i) the generation of high-quality biomarker data via the fully automated Elecsys® platform;
(ii) a digital research environment using the NTKApp, which is fully integrated on ADDI’s AD Workbench.
The NTK is an innovative approach to accelerate the validation of biomarkers for clinical utility and the best-in-class tool for collaborative data analysis; the purpose of the measurements is not clinical decision-making.
Vision
| Mission
|
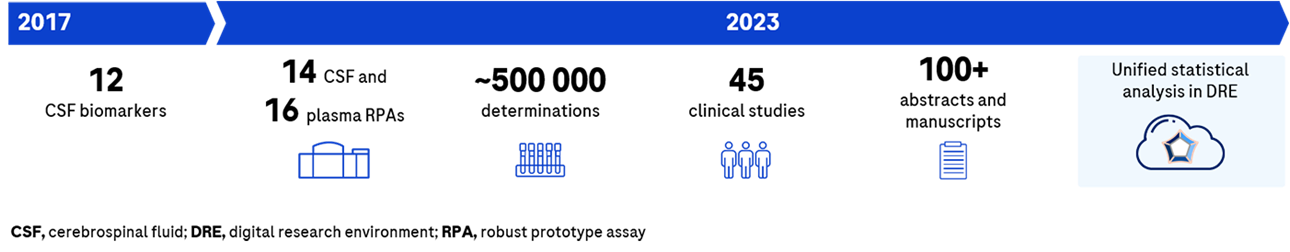
Timeline
Categories
Robust Prototype Assay Portfolio
The NTK offers an intended-use agnostic portfolio of 14 CSF and 16 serum/plasma assays, with more in development.
The NTK assays can be run on the fully automated Elecsys® platform in four NTK qualified labs. This ensures that high quality, reproducible data can be compared to multiple datasets, thus maximizing how results can be interpreted.
NTKApp
The NTKApp is a powerful, cloud based, fully integrated analytics platform. It improves the process and ability to interpret neurologically relevant immunoassay data while providing users a unified, cloud-based platform. The NTKApp consists of three interlinked apps to upload, curate, analyze, and compare biomarker datasets and results. Thanks to the NTKApp built-in virtual machine, we offer the option to import, shape, and run your own statistical analysis pipeline that can later be shared with the NTK community. The NTKApp is a new tool to exchange new ideas, encourage valuable research, and support industry partnerships – all to accelerate biomarker discovery and development.
How to become a partner
If you are interested in joining the NTK project, please reach out to:
Margherita Carboni (margherita.carboni@roche.com), Clinical Development Lead
Karolina Jarawka (karolina.jarawka@roche.com), Director Partenering
After your request is evaluated, you will have to sign an NTK collaboration agreement, including a material transfer agreement with the designated NTK testing site.
A fee per determination (one sample measured with one biomarker) is charged per cohort. This covers all costs, including reagents and lab fees.
If you have any generic requests or questions about the NeuroToolkit, please reach out to neurotoolkit@roche.com.
Publications
- Identifying clinically useful biomarkers in neurodegenerative disease through a collaborative approach: The NeuroToolKit - January 2023
- Optimal combinations of CSF biomarkers for predicting cognitive decline and clinical conversion in cognitively unimpaired participants and mild cognitive impairment - January 2023
- Liver-specific polygenic risk score is associated with Alzheimer’s disease diagnosis - January 2023
- Neuropathology-based APOE genetic risk score better quantifies APOE effect in statistical analyses - February 2023
- The recency ratio assessed by story recall is associated with cerebrospinal fluid levels of neurodegeneration biomarkers - February 2023
- Biological brain age prediction using machine learning on structural neuroimaging data: multi-cohort validation against biomarkers of Alzheimer's disease and neurodegeneration stratified by sex - April 2023
- Identification of plasma metabolites associated with modifiable risk factors and biomarkers reflecting Alzheimer’s disease pathology - March 2023
- Large-scale proteome and metabolome analysis of CSF implicates altered glucose metabolism and succinylcarnitine in Alzheimer’s disease - May 2023
- Asthma and the lung-brain axis in cognitive decline: evidence from diffusion MRI and CSF biomarkers - June 2023
- Prevalence and Clinical Implications of a β-Amyloid–Negative, Tau-Positive Cerebrospinal Fluid Biomarker Profile in Alzheimer Disease - July 2023
- CSF sphingomyelins in Alzheimer’s disease, neurodegeneration, and neuroinflammation - January 2022
- Age, Sex and APOE-e4 modify the balance between soluble and deposited b-amyloid in cognitively intact individuals: topographical patterns and replication across two independent cohorts - January 2022
- Crosswalk study on blood collection-tube types for Alzheimer’s disease biomarkers - February 2022
- Test-retest variability of plasma biomarkers in Alzheimer’s disease and effects on clinical prediction models - April 2022
- Brain alterations in the early AD continuum with amyloid, tau, glial and neurodegeneration CSF markers - May 2022
- Inflammation, tau pathology, and synaptic integrity disrupt sleep spindles and memory prior to B-amyloid positivity - June 2022
- Identification of plasma metabolites associated with modifiable risk factors and biomarkers reflecting Alzheimer’s disease pathology - June 2022
- Reactive astrogliosis is associated with higher cerebral glucose consumption in the early Alzheimer’s continuum - June 2022
- Asthma amplifies dementia risk: evidence from CSF biomarkers and cognitive decline - July 2022
- Principal components from untargeted CSF metabolomics associated with AD biomarkers - September 2022
- Pre-pandemic Alzheimer's disease biomarkers and anxious-depressive symptoms during the COVID-19 confinement in cognitively unimpaired adults - October 2022
- Genetically predicted telomere length and Alzheimer's disease endophenotypes: a Mendelian randomization study - November 2022
- CSF sphingomyelins in Alzheimer’s disease, neurodegeneration, and neuroinflammation - November 2022
- Associations between semantic memory for proper names in story recall and CSF amyloid and tau in a cognitively unimpaired sample - December 2022
- Associations Between Diffusion MRI Microstructure and Cerebrospinal Fluid Markers of AD Pathology and Neurodegeneration along the AD Continuum - December 2022
- Association between air pollution exposure and biomarkers of Alzheimer’s disease: A cross-sectional study in at risk cognitively unimpaired individuals - December 2021
- CSF Synaptic Biomarkers in the Preclinical Stage of Alzheimer Disease and Their Association With MRI and PET: A Cross-sectional Study - November 2021
- Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum - October 2021
- Enhancing the Sensitivity of Memory Tests: Reference Data for the Free and Cued Selective Reminding Test and the Logical Memory Task from Cognitively Healthy Subjects with Normal Alzheimer’s Disease Cerebrospinal Fluid Biomarker Levels - September 2021
- Large-scale proteome analysis of CSF implicates altered glucose metabolism in Alzheimer’s disease - September 2021
- Amyloid positive individuals with subjective cognitive decline present increased CSF Neurofilament Light levels that relates to hippocampal volume - August 2021
- Perivascular spaces are associated with tau pathophysiology and synaptic dysfunction in early Alzheimer’s continuum - August 2021
- Insulin resistance is related to cognitive decline but not change in CSF biomarkers of Alzheimer’s disease in non-demented adults - July 2021
- Cognitively unimpaired individuals with low burden of Abeta pathology have a distinct CSF biomarker profile - July 2021
- Liver-specific polygenic risk score is more strongly associated than genome-wide score with Alzheimer’s disease diagnosis - May 2021
- Interaction of amyloid and tau on cortical microstructure in late middle-aged cognitively unimpaired adults - May 2021
- Cerebral amyloid-β burden is associated with neurodegeneration and gliosis: Mediation by p-tau and interactions with risk factors early in the Alzheimer's continuum - March 2021
- Liver-specific polygenic risk score is more strongly associated than genome-wide score with Alzheimer’s disease diagnosis - March 2021
- Association of weight change with cerebrospinal fluid biomarkers and amyloid positron emission tomography in preclinical Alzheimer’s disease - February 2021
- Novel tau biomarkers phosphorylated at T181, T217 or T231 increase in initial stages of preclinical Alzheimer's continuum when only subtle changes of Ab pathology are detected - December 2020
- Comparative analysis of different definitions of amyloid-β positivity to detect early downstream pathophysiological alterations in preclinical Alzheimer - September 2020
- An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum - July 2020
- Beta Amyloid, Tau, synaptic, neurodegeneration and glial biomarkers in the preclinical stage of the Alzheimer’s continuum - June 2020
FAQ's
The NeuroToolKit (NTK) is the result of an academic and industry partners to generate high quality, reproducible, and comparable biomarker data. Research collaboration will be instrumental in generating evidence to clarify the use of biomarkers in clinical trials and routine clinical practice. This should accelerate the development of In Vitro Diagnostics (IVD) biomarkers that: (1) support clinical screening, diagnosis, and prognosis, (2) track pharmacodynamic responses, and (3) monitor drug efficacy. Roche Diagnostics uses the collaborative NTK approach to accelerate and inform decision-making regarding the clinical utility and IVD development of medical value assays.
The NTK offers a portfolio of exploratory prototype assays designed to generate high-quality comparable clinical biomarker data across academic and industry cohorts. The NTK assays can be run on the fully automated Elecsys® platform in four NTK qualified labs. This ensures that high quality, reproducible data can be compared to multiple datasets - maximizing how results can be interpreted. By clarifying the clinical utility of biomarkers and how they can be used in clinical trials, it identifies and supports those biomarkers which need to be developed further.
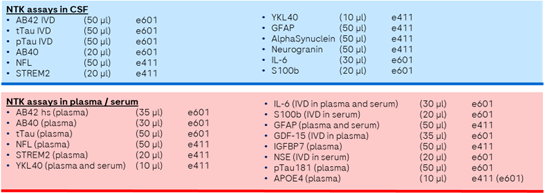
The NTK portfolio for CSF currently includes robust prototype assays to measure any combination of the following biomarkers: Aβ42 (IVD ), Aβ40, t-tau (IVD ), p-tau181 (IVD ), neurogranin, GFAP, IL-6, YKL-40, sTREM2, NfL, S100B and α-synuclein.
The NTK portfolio for plasma currently includes robust prototype assays to measure any combination of the the following biomarkers:s Aβ42 high sensitive, Aβ40, tTau, NfL, sTREM2, YKL-40, IL-6 (IVD), S100B (IVD in serum), GFAP, GDF-15 (IVD in plasma in development), IGFBP7, NSE IVD in serum) pTau181 (IVD in plasma in development), APOE4 (IVD in plasma in development) .
Additional biomarkers that will soon be available: TDP 43 (serum), SNAP 25 (CSF/plasma), NPTX 2 (CSF/plasma).
The required volume depends on the selected biomarker to be measured and sample type. Please see below for more details.
The exploratory prototype assays are for human research only (for basic research or for early investigation of potential clinical utility of biomarkers). Measurements made with the NTK robust prototype assays must not be used for any medical and/or clinical decision making.
We have a strong foundation of reliable, comparable and high quality biomarker data, which highlights the benefits of using the Elecsys® technology in neurological diagnostics.
Although amyloid plaque deposition and tau tangles are the main pathological hallmarks of the disease, neuroinflammation, microglial overactivation and axonal/synaptic neurodegeneration to play an important role in AD pathogenesis. These pathological processes are complementary to the amyloid cascade hypothesis and are reflected by changes in biomarker levels measured in the NTK panel.
Similar to AD, neurodegenerative diseases such as Parkinson’s disease and Multiple Sclerosis involve a series of pathological processes that include synaptic and axonal degeneration, inflammation and microglial overactivation. Assessing specific markers of each disease's pathological process may help explore potential biomarker disease profiles to reach a differential diagnosis.
The NTKApp is a cloud based data analytics tool to curate, analyze and compare biomarker datasets and results - maximizing the interpretability of the generated results. It is deployed on the AD Workbench, a trusted cloud based platform for collaborative data science.
The NTKApp consists of three interlinked apps. This allows users to upload and curate, analyze, and compare biomarker datasets and results from all over the world in a few simple clicks. Thanks to the NTKApp built-in virtual machine, users have the option to import, shape, and run their own statistical analysis pipeline, which can later be shared within the NTK community.
The NTKCuration App module offers user-friendly data upload. Biomarker data needs quality control and standardization before being used in the NTKAnalysis App. - The NTKCuration App saves time and money because this automated data standardization process minimizes human error and allows comparability across datasets.
The NTKAnalysis App allows users to select from a suite of powerful descriptive statistics to gain immediate insight into their data. Outputs from these analyses are available in publication-quality tables and interpretable graphical visualizations, which can be saved locally.
The NTKMeta-Analysis App allows biomarker data to be compared with other data across the community. The cloud can be harnessed to select, stratify, and compare biomarkers and clinical characteristics in a global way. The NTKMeta-Analysis App also sheds light on shared data, resources, and objectives, providing support for existing and new clinical use cases based on collective evidence. This enables results to be contextualized and new hypotheses and evidence to be generated.
The NTKApp is a new neurological tool to exchange new ideas, encourage valuable research, and support industry partnerships – all to accelerate biomarker discovery and development.
To become a member and to access NTK measurements, please reach out to Roche Diagnostics. You will need to complete an access form and submit an access fee. Once your access request is approved, you will need to sign an amended agreement, a materials transfer agreement with the NTK site, and submit a peer-determination fee.
For academic inquiries, please contact Margherita Carboni, Clinical Scientist - Neurology (margherita.carboni@roche.com). For industry inquiries, please contact Karolina Jarawka, Director Partnering (karolina.jarawka@roche.com).
No, measurements have to be performed in one of the four qualified NTK laboratories: LabCorp Indianapolis (USA), University of Gothenburg (Sweden), Microcoat (Penzberg, Germany) and TrigaS (Habach, Germany).